16 November 2023
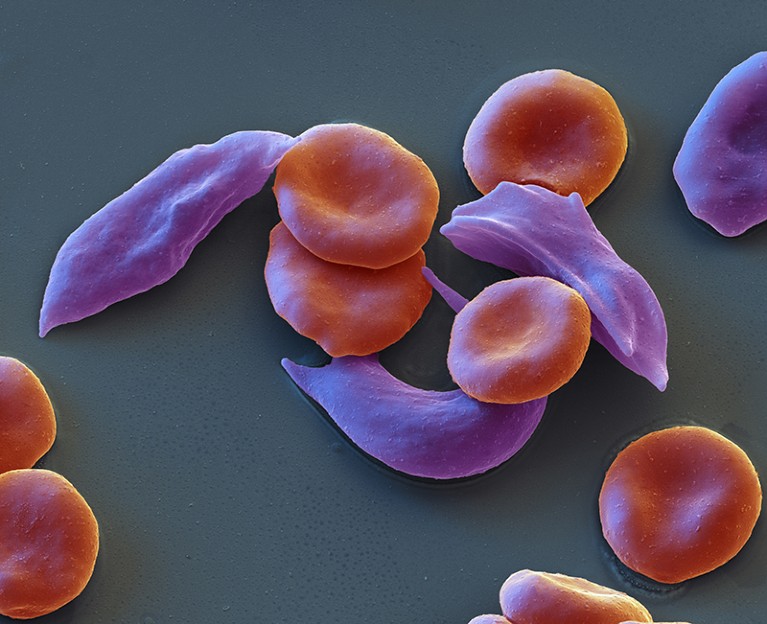
The landmark decision could transform the treatment of sickle-cell disease and β-thalassaemia — but the technology is expensive.
In a world first, the UK medicines regulator has approved a therapy that uses CRISPR gene editing as a treatment for diseases. The decision marks another high point for a biotechnology that has regularly been lauded as revolutionary in the decade since its discovery.
The therapy, called Casgevy, will treat the the blood conditions sickle-cell disease and β-thalassaemia. Sickle-cell disease, also known as sickle-cell anaemia, can cause debilitating pain, and people with β-thalassaemia can require regular blood transfusions.
“This is a landmark approval which opens the door for further applications of CRISPR therapies in the future for the potential cure of many genetic diseases,” said Kay Davies, a geneticist at the University of Oxford, UK, in comments to the UK Science Media Centre.
Nature explains the research behind the treatment and explores what’s next.
-
Tags
#United Kingdom